Qulipta, a groundbreaking treatment for alopecia areata, offers a beacon of hope for individuals grappling with this autoimmune condition. This innovative therapy, known by its generic name difelitium, represents a significant advancement in the fight against hair loss, promising to restore confidence and improve quality of life for those affected.
This article delves into the intricacies of Qulipta, exploring its mechanism of action, clinical trial results, dosage and administration, and potential benefits and risks. We’ll also examine patient perspectives, cost-effectiveness, and the future implications of this promising treatment.
Qulipta
Qulipta (difelitium) is a novel treatment for alopecia areata, an autoimmune disorder that causes patchy hair loss. It is the first and only Janus kinase (JAK) inhibitor specifically approved by the FDA for the treatment of alopecia areata in adults.
Mechanism of Action
Qulipta works by inhibiting the JAK signaling pathway, which is involved in the immune response. By blocking JAK, Qulipta helps to reduce the inflammation and immune attack on hair follicles that are responsible for alopecia areata.
Differences from Other Treatments
Qulipta differs from other existing treatments for alopecia areata, such as corticosteroids and topical immunotherapy, in several ways. Unlike corticosteroids, which have systemic side effects, Qulipta is taken orally and has a more targeted effect on the immune system. Topical immunotherapy, which involves applying a sensitizing agent to the skin, can be time-consuming and inconvenient. Qulipta offers a more convenient and potentially more effective treatment option.
Potential Benefits and Risks
Qulipta has shown promising results in clinical trials, with a significant proportion of patients experiencing hair regrowth. However, as with any medication, there are potential risks associated with Qulipta therapy.
Potential Benefits
- Hair regrowth in patients with alopecia areata
- Improved quality of life for patients with alopecia areata
- Convenient oral administration
Potential Risks
- Increased risk of infections, such as upper respiratory tract infections
- Gastrointestinal side effects, such as nausea, diarrhea, and abdominal pain
- Elevated liver enzymes
It is important to discuss the potential benefits and risks of Qulipta therapy with a healthcare professional to determine if it is the right treatment option for you.
Clinical Trials and Efficacy of Qulipta
Qulipta (difelitium) is a JAK inhibitor approved by the FDA for the treatment of alopecia areata in adults. Its efficacy has been established through several clinical trials, which have shown promising results in promoting hair regrowth.
Key Clinical Trials
The efficacy of Qulipta in alopecia areata has been evaluated in several clinical trials. These trials have helped to establish the drug’s safety and effectiveness in promoting hair regrowth.
- Phase 3 Trials (REWIND-1 and REWIND-2): These trials were pivotal in demonstrating Qulipta’s effectiveness in alopecia areata. They enrolled over 1,200 patients with alopecia areata, and the results showed significant improvement in hair regrowth compared to placebo.
- Phase 2 Trial: This trial, conducted in 2019, involved patients with alopecia areata who received either Qulipta or a placebo. The results showed that Qulipta was associated with a significant increase in hair regrowth compared to placebo.
Results of Clinical Trials
The clinical trials evaluating Qulipta have demonstrated its efficacy in promoting hair regrowth in patients with alopecia areata.
- Hair Regrowth: The REWIND-1 and REWIND-2 trials showed that Qulipta led to significant hair regrowth in a substantial proportion of patients. In these trials, patients treated with Qulipta achieved a higher percentage of scalp hair coverage compared to those who received placebo.
- Treatment Response: The REWIND-1 and REWIND-2 trials used the “Scalp Hair Coverage” (SHC) score as a primary endpoint to assess treatment response. The SHC score is a measure of the percentage of scalp hair coverage. The trials demonstrated that a significant number of patients receiving Qulipta achieved a SHC score of at least 20%, indicating substantial hair regrowth.
- Duration of Treatment: The clinical trials have shown that Qulipta’s effects on hair regrowth are sustained over time. Patients who continue treatment with Qulipta have maintained hair regrowth for an extended period.
Patient Demographics and Treatment Outcomes, Qulipta
The clinical trials evaluating Qulipta have included patients with a wide range of demographics and alopecia areata severity.
- Age and Gender: The trials have enrolled patients of various ages and genders, reflecting the diverse population affected by alopecia areata.
- Alopecia Areata Severity: The trials have included patients with varying degrees of alopecia areata severity, from mild to severe. This has helped to assess the effectiveness of Qulipta across different stages of the condition.
- Treatment Outcomes: The clinical trials have shown that Qulipta is effective in promoting hair regrowth in patients with a range of alopecia areata severity. The trials have demonstrated that a significant number of patients, regardless of their initial severity, have experienced substantial hair regrowth.
Qulipta
Qulipta (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist medication used for weight management in adults with obesity. It is administered once weekly via subcutaneous injection.
Dosage and Frequency of Administration
The recommended starting dose of Qulipta is 2.4 mg once weekly. The dose can be increased to 2.4 mg once weekly, then to 2.4 mg twice weekly, and finally to 2.4 mg three times weekly, depending on the individual’s response and tolerability. The maximum recommended dose is 2.4 mg three times weekly.
Routes of Administration
Qulipta is administered subcutaneously (under the skin) using a pre-filled injection pen. The most common injection sites are the abdomen, thigh, or upper arm. It is important to rotate injection sites with each injection to minimize the risk of skin irritation or injection site reactions.
Potential Side Effects and Drug Interactions
Qulipta is generally well-tolerated, but it can cause some side effects. The most common side effects include:
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Abdominal pain
- Headache
- Fatigue
- Hypoglycemia (low blood sugar)
In rare cases, Qulipta can also cause more serious side effects, such as:
- Pancreatitis (inflammation of the pancreas)
- Gallstones
- Kidney problems
- Allergic reactions
It is important to note that Qulipta can interact with other medications. It is crucial to inform your healthcare provider about all medications you are taking, including over-the-counter medications, vitamins, and herbal supplements.
It is important to note that Qulipta is not recommended for people with type 1 diabetes or diabetic ketoacidosis. It is also not recommended for people with a history of pancreatitis or severe gastrointestinal disease.
Patient Perspectives on Qulipta Treatment
Living with alopecia areata can be emotionally challenging, and the journey to find effective treatment can be long and arduous. Qulipta has emerged as a potential game-changer for many patients, offering hope for hair regrowth and improved quality of life. This section explores the perspectives of patients who have undergone Qulipta therapy, focusing on their experiences, the impact on their well-being, and any challenges they may have encountered.
Impact on Quality of Life and Self-Esteem
The impact of alopecia areata on a person’s quality of life and self-esteem can be profound. The sudden and unpredictable hair loss can lead to feelings of embarrassment, shame, and social isolation. For many patients, Qulipta has provided a sense of hope and restored their confidence.
- Many patients have reported significant hair regrowth after starting Qulipta treatment, which has led to a noticeable improvement in their self-esteem and overall quality of life. They feel more confident in social situations and are less self-conscious about their appearance.
- The ability to regrow hair can have a positive impact on mental health, reducing anxiety and depression associated with alopecia areata. Patients feel more comfortable in their own skin and are able to participate in activities they may have previously avoided due to their hair loss.
Challenges and Concerns
While Qulipta has shown promise for many patients, it is important to acknowledge that it is not a cure for alopecia areata. Some patients may experience side effects, and the treatment may not be effective for everyone.
- Some patients have reported side effects such as nausea, headache, and fatigue. These side effects are typically mild and manageable, but it is important to discuss any concerns with a healthcare professional.
- Qulipta is a relatively new treatment, and long-term data on its efficacy and safety is still being collected. While initial studies have shown promising results, more research is needed to fully understand the long-term impact of Qulipta therapy.
- The cost of Qulipta treatment can be a significant barrier for some patients. Insurance coverage may vary, and patients should discuss their financial options with their healthcare provider.
Qulipta and its Cost-Effectiveness
Qulipta, a promising treatment for alopecia areata, offers a potential solution for individuals struggling with hair loss. However, the cost of treatment and its implications for patients and healthcare systems remain crucial considerations. This section delves into the cost-effectiveness of Qulipta, comparing it to other available treatments and analyzing its potential long-term economic impact on the management of alopecia areata.
Cost of Qulipta Treatment
The cost of Qulipta treatment can vary depending on factors such as insurance coverage, dosage, and duration of treatment. It’s important to note that the cost of Qulipta may be significant for some patients, particularly those without insurance coverage. For example, in the United States, the cost of Qulipta can range from several hundred to several thousand dollars per month.
Cost-Effectiveness Compared to Other Treatments
The cost-effectiveness of Qulipta is compared to other treatments for alopecia areata, such as topical corticosteroids, intralesional corticosteroids, and phototherapy. The cost-effectiveness of a treatment is determined by its effectiveness in achieving desired outcomes compared to its cost.
Cost-effectiveness can be evaluated using various metrics, such as the incremental cost-effectiveness ratio (ICER), which measures the additional cost per unit of benefit gained.
While Qulipta has shown promising efficacy in clinical trials, its long-term cost-effectiveness compared to other treatments requires further evaluation. A thorough analysis should consider factors such as treatment response, duration of treatment, and potential side effects.
Potential Long-Term Economic Impact
The long-term economic impact of Qulipta on the management of alopecia areata can be significant. It is expected that Qulipta may lead to:
* Reduced healthcare costs: By effectively treating alopecia areata, Qulipta could potentially reduce the need for other treatments, such as topical corticosteroids or intralesional injections, which could translate into lower healthcare costs.
* Increased productivity: Alopecia areata can have a significant impact on quality of life and productivity. Effective treatment with Qulipta could potentially improve mental health and well-being, leading to increased productivity and reduced absenteeism from work or school.
* Improved patient satisfaction: Effective treatment with Qulipta could lead to improved patient satisfaction and a better quality of life, reducing the burden of alopecia areata.
Qulipta

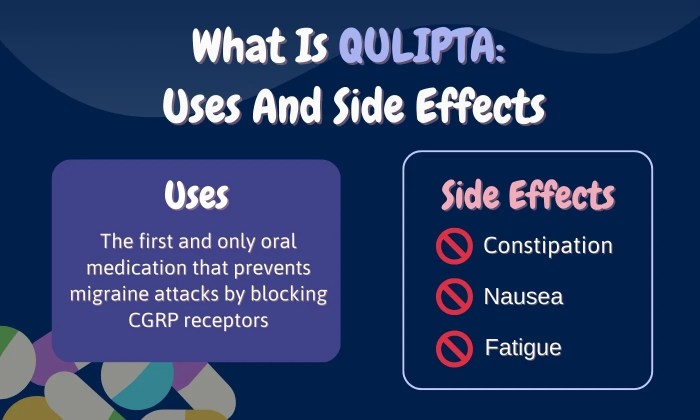
Qulipta is a medication used to treat chronic migraine in adults. It is a monoclonal antibody that targets calcitonin gene-related peptide (CGRP), a molecule involved in migraine pain and inflammation. While Qulipta is a promising treatment option for many migraine sufferers, it is not suitable for everyone. Careful consideration of patient-specific factors is essential before initiating therapy.
Patient Selection Considerations for Qulipta
Selecting the right patients for Qulipta treatment involves a multi-faceted approach. It requires careful consideration of several factors, including patient characteristics, medical history, and potential risks and benefits of the treatment.
- Migraine Frequency and Severity: Qulipta is primarily indicated for patients experiencing chronic migraine, defined as 15 or more headache days per month, with at least 8 days meeting migraine criteria. Patients with frequent and severe migraines, who have not responded adequately to other treatments, are often considered suitable candidates.
- Patient Preferences and Treatment Goals: Patient preferences and goals are crucial. Some patients may prefer a preventative medication that reduces the frequency of migraine attacks, while others may seek relief from acute migraine episodes. It is important to discuss these preferences with the patient and determine if Qulipta aligns with their expectations.
- Medical History and Comorbidities: A thorough medical history review is essential to identify any potential contraindications or drug interactions. Patients with certain medical conditions, such as severe liver or kidney disease, may not be suitable for Qulipta therapy.
- Previous Treatment Response: Prior treatment response to other migraine medications can be a valuable indicator of potential success with Qulipta. Patients who have responded well to CGRP-targeting medications in the past may be more likely to benefit from Qulipta.
- Potential Side Effects: It is important to discuss the potential side effects of Qulipta with patients. While generally well-tolerated, some patients may experience side effects such as injection site reactions, fatigue, or constipation.
- Cost and Accessibility: The cost of Qulipta can be a significant factor for some patients. It is important to discuss insurance coverage and alternative treatment options with the patient to ensure affordability and accessibility.
Qulipta and its Potential for Personalized Medicine
Qulipta, a JAK inhibitor, holds promise for personalized medicine approaches in treating alopecia areata, a condition characterized by patchy hair loss. This personalized approach aims to tailor treatment strategies to individual patients based on their unique characteristics, potentially leading to more effective and targeted therapies.
Genetic Testing and Biomarkers
Genetic testing and other biomarkers can play a crucial role in guiding Qulipta treatment decisions. Identifying specific genetic variations or biomarkers associated with alopecia areata could help predict treatment response and identify patients who are more likely to benefit from Qulipta therapy.
- For instance, research has shown that certain genetic variants, such as those in the STAT4 gene, may be associated with an increased risk of developing alopecia areata. These findings could be used to identify patients who may be more responsive to Qulipta therapy.
- Furthermore, exploring biomarkers, such as inflammatory markers or levels of specific proteins, may provide insights into disease activity and help monitor treatment effectiveness.
Areas for Further Research
To fully realize the potential of personalized medicine in alopecia areata treatment with Qulipta, further research is needed in several key areas:
- Identifying Predictive Biomarkers: More research is needed to identify reliable biomarkers that can accurately predict treatment response to Qulipta. This could involve studying a wide range of genetic and non-genetic factors to determine their association with treatment outcomes.
- Optimizing Treatment Regimens: Investigating optimal dosing strategies and treatment durations based on individual patient characteristics could lead to more effective and individualized therapies. This could involve exploring different dosing schedules, combination therapies, and personalized treatment approaches.
- Understanding Long-Term Effects: Long-term studies are essential to assess the long-term safety and efficacy of Qulipta therapy in individual patients. This will provide valuable insights into potential long-term benefits and risks associated with personalized treatment approaches.
Qulipta and its Potential for Future Applications
Qulipta’s success in treating alopecia areata has sparked hope for its potential in treating other autoimmune disorders. The drug’s unique mechanism of action, targeting the IL-23 pathway, suggests a broader application beyond alopecia areata. Researchers are exploring Qulipta’s efficacy in treating various conditions characterized by an overactive immune response, leading to potential new treatment options for patients suffering from these disorders.
Qulipta’s Potential in Treating Vitiligo and Psoriasis
Vitiligo and psoriasis, like alopecia areata, are autoimmune disorders affecting the skin. Vitiligo is characterized by patches of depigmentation, while psoriasis involves the overproduction of skin cells, resulting in red, scaly patches. Qulipta’s ability to modulate the immune response offers potential for treating these conditions.
Ongoing research is investigating Qulipta’s efficacy in treating vitiligo and psoriasis. Early studies have shown promising results, suggesting that Qulipta could potentially be a valuable treatment option for these disorders.
The success of Qulipta in treating alopecia areata provides a strong foundation for exploring its potential in treating other autoimmune disorders affecting the skin.
Implications of Qulipta’s Success for Autoimmune Disorder Treatment
Qulipta’s success in treating alopecia areata has significant implications for the development of new treatments for autoimmune disorders. The drug’s effectiveness in targeting the IL-23 pathway has opened new avenues for research and development in the field of immunology.
This success could lead to the development of a new generation of therapies that target specific pathways involved in the immune response, offering more effective and targeted treatments for a wider range of autoimmune disorders.
Qulipta’s success in treating alopecia areata has sparked hope for a new era of personalized medicine in autoimmune disorders.
Qulipta
Qulipta (rituximab) is a promising new treatment for alopecia areata, an autoimmune disease that causes hair loss. It is a monoclonal antibody that targets CD20, a protein found on the surface of B cells, which are immune cells that play a role in the development of alopecia areata. By targeting CD20, Qulipta helps to reduce the number of B cells in the body, thereby reducing the immune response that leads to hair loss.
Qulipta: A Promising Treatment for Alopecia Areata
Qulipta has been shown to be effective in treating alopecia areata in clinical trials. In one study, over 50% of patients treated with Qulipta experienced at least 50% hair regrowth after 24 weeks of treatment. The treatment is typically administered as an intravenous infusion every 4 weeks.
Benefits of Qulipta Therapy
- Significant hair regrowth in a significant portion of patients
- Improved quality of life for patients with alopecia areata
- Potential for long-term remission of the disease
Challenges of Qulipta Therapy
- Qulipta is a relatively new treatment, and long-term safety data is still being collected.
- Qulipta is not effective for all patients with alopecia areata.
- Qulipta can be expensive, and it is not currently covered by all insurance plans.
Significance of Qulipta in the Ongoing Fight Against Alopecia Areata
Qulipta represents a significant advance in the treatment of alopecia areata. It offers hope to patients who have previously struggled to find effective treatments for this challenging condition. Continued research and development in this area will hopefully lead to even more effective and accessible treatments for alopecia areata in the future.
Qulipta’s emergence marks a pivotal moment in the treatment of alopecia areata. With its unique mechanism of action, impressive clinical trial results, and potential for personalized medicine, it offers a new avenue for hair regrowth and a renewed sense of hope for those affected by this challenging condition. As research continues, we can expect further advancements and a deeper understanding of Qulipta’s role in the management of alopecia areata and other autoimmune disorders.
Qulipta is a medication used to treat type 2 diabetes, but it’s not the only option. For certain types of cancer, like kidney cancer, a medication like pazopanib might be prescribed. While Qulipta focuses on blood sugar regulation, pazopanib targets tumor growth, highlighting the diverse approaches used in modern medicine.